
[HOME PAGE] [CURRICULUM VITAE] [DRAFTS AND SKETCHES]
26 January 2003 - http://forums.about.com/ab-christianity/messages?msg=10917.2462
Given that Ernst Mayr's recent book and S.J. Gould's last book The Structure of Evolutionary are being discussed on this thread, and given my personal knowledge of the authors and their subjects, I suppose I should make some comments in the first person. My take on these books from the religious point of view is that they provide all the ammunition required to show that there is absolutely no requirement for supernatural guidance to account for the diversity of living things we see in the world today. I will return to this point in my conclusion.
However, both authors take a somewhat narrow approach to evolution, and lack much in the way of first hand experience with just how diverse life is. To have this one needs a deep background in population biology, genetics and cytogenetics, and a familiarity with the entire lifecycles of the teeming numbers of very small to microscopic organisms living in marine habitats as well as macroscopic shelly moluscs and vertebrates.
In what follows I touch on some of these threads, and give some examples of why I think the creationist and "intelligent" design approaches to explaining the diversity of life are so vapid and devoid of any genuine basis in reality.
By way of background on my comments on the books and the Christianity threads, I studied under both Mayr and Gould when working on my PhD at Harvard in the Museum of Comparative Zoology, which I completed in 1973. Mayr was a member of my thesis committee, and as Darwinia noted on my behalf, Gould was then his non-tenured his assistant in the Evolutionary Biology course. My major advisor was Ernest Williams, the herpetologist whose work centered on population biology and evolution of the West Indian Anolis lizards, who was probably the best advisor Harvard in evolutionary biology, and who certainly had the most students.
My own thesis was on the population cytogenetics, evolution and speciation of the iguanid lizard genus Sceloporus. Fence swifts are the most common Sceloporus through most of the US, with a number of additional species extending into the southwestern states. The radiation of the genus is based around the Mexican Plateau, with approximately 60 species being recognized taxonomically when I completed my thesis. Several incipient species are at intermediate stages of evolving reproductive isolation, meeting in hybrid zones a few hundred meters wide (where intermediate forms are found, but apparently being genetically isolated (as show by the failure of certain genetic markers to cross the hybrid zone). My primary focus was on understanding the evolutionary mechanics of how reproductive isolation could have evolved at the population genetic and chromosomal level without the populations having been separated geographically. The thesis also involved a plausible reconstruction of the overall radiation of the existing species from a common ancestor, including an analysis of all the geographic and intrinsic (i.e., chromosomal mutation) factors likely to have been involved in the speciation events.
Mayr and Gould both approach the study of evolution from the level of descriptive systematics (taxonomy and classification) working primarily with dead and preserved remains of organisms, with Gould offering the additional experience of paleontology and the fossil record. By comparison to their backgrounds in "classical" museum systematics, I worked primarily with organisms in the field, in life, and had the added advantages (1) of growing up on the ocean front in Southern California, where I spent a respectable amount of my youth immersed in a variety of different littoral habitats ranging from the kelp beds and tidepools of Catalina Island to pilings, floats and mudflats of smelly estuaries, and (2) having access to a good quality compound microscopes from the time I was 8 or 9 years old to see things too small to be resolved with the unaided eye. Beyond the early experience with marine diversity, and my research experience with vertebrate speciation, I have had around 12 years experience teaching courses in invertebrate zoology, comparative anatomy (I had already taught these courses at Southern Illinois University Edwardsville BEFORE I started my PhD work at Harvard), vertebrate zoology, marine biology, genetics, zoogeography, cytogenetics, and evolutionary biology, where we did as much work as possible with living organisms. In my three years teaching at the University of Puerto Rico in San Juan, my courses included frequent lab field trips to skindive on local coral reefs where we paid particular attention to invertebrates (until in my last year the best sites were destroyed by runoff from construction sites). We also maintained several aquaria in the lab where I mounted stereo microscopes able to look into the microcosms through the sides of the tanks in order to see some of the true diversity of marine life.
Although I am currently practicing as a documentation systems analyst and knowledge manager in the defense industry and part time back in academia as a knowledge management research fellow, I have not lost my interest in evolutionary biology or understanding of the subject. My change of field was primarily for economic reasons and the perversions of academia in the US under affirmative action. I graduated at the tail end of the Baby Boom with declining enrollment and as a WASP with a complex resume: At college, I majored in physics for 3-1/2 years before flunking out. I completed my BS in Zoology, did two years master's degree work at Southern Illinois University, Edwardsville before they had a master's degree program, finishing up with my PhD at Harvard. I managed to stay in academia for another 7 years after graduating - which included two years of postdoctoral work in the late 70's here in Melbourne studying the history and philosophy of science to resolve my understanding of the comparative approach, but I never managed to score a good tenure track position that allowed for both teaching and research. In Australia around 1981 I discovered that personal computers were evolving many millions of times faster than my lizards, and after entering the computer industry as a computer literacy educator and trainer, I have retooled myself as an agent of change in this industry.
Now, back to the books and their limitations: As Darwinia noted in an earlier post, I have yet to see Mayr's latest (much of what I know about major features of evolution I learned from Mayr's earlier tomes - so I can certainly recommend the book), but I am well into Gould's. At this point - although I suspect I will have many quibbles with the details, I am prepared to accept that Gould's Structure of Evolutionary Theory is the pinnacle of what the "fundies" tend to call "macro"evolutionary theory. For example based on my own extensive reading of Darwin and works on Darwin, Gould's Chapter 2 on "The Essence of Darwinism and the Basis of Modern Orthodoxy: An Exegesis of the Origin of Species" is by far the most complete and clearest logical explanation of how evolution works I have found. If you accept Gould's premises (and there is certainly no evidence to refute them) evolution is the logically inescapable conclusion. Gould absolutely accepts this as the bedrock and foundation for all evolutionary theory, but will spend much of the remainder of the book extending the basic structure to account for what he sees as limitations in the predictions of the orthodox theory in terms of accounting for non-linear aspects of the paleontological record. Here, is where I will probably have some disagreements with Gould based on our very different viewpoints on the history and diversity of life. Personally, I see no reason not to accept the orthodox theory as is - without the need for any major extensions to explain "punctuated evolution". [October 2004 Note that when I wrote this critique I was trying to wade through Gould's tome and was very angry with Gould's verbosity, where he uses three times as many words as needed to make a point. Although the book is arm-breakingly ponderous, filled with pompous pretentious prose, and does not adequately consider the population genetic or cytogenetic mechanics of evolution, it is nevertheless a masterwork of historical and critical analysis capping the whole of 20th Century evolutionary thinking.]
Gould's experience was primarily with shells - i.e., hard remains of adult organisms preserved in the fossil record. To him, 10,000 years is an instant. Although he spent several years in the field in New Guinea and the Pacific islands, Mayr worked primarily with bird skins in museum cases. My major criticism of the books is that they look at phenomenon of evolution from a relatively superficial level. Even deeper analysis provides far more support for evolution as a totally natural process as opposed to a god mediated supernatural process.
I have studied rapidly evolving lizards in the field - where at least some of the species with a 2 year generation time have probably been formed since the last ice age (i.e., around 6,000 generations) and studied genetic variation in natural populations and species borders down the the dispersal migrations of single individuals.
To give some feeling for Gould's long term view of evolution, I observe that probably 95% of species have a generation time of a year or less. Even with this, Gould is concerned that classical selection (i.e., differential survival and reproduction of individual organisms in a species' population) is too slow to account for the observed patterns of diversity. I counter by observing that at least for most marine species (where essentially all of the major body plans evolved), individuals of many of the species produce hundreds of thousands to millions of fertilized eggs for the expectation that only one will survive to replace the egg producing parent, and I do not forget that selection works on all stages of the lifecycle from the point where the gametes are released into the environment to the time the zygote produces the next generation of gametes into the environment.
In most species, selection on larvae is probably much stronger than that on the adult form. Most experimentation with basic body plans has probably involved selection at the larval stage to improve the chances of larval survival through improved mobility and feeding capabilities. This is actually a very interesting phenomenon that is practically unknown outside of the discipline of invertebrate zoology. Most sessile organisms (e.g., plants, sponges, corals and other coelenterates, bryzoa, tunicates and the like) produce free-living mobile larvae who have the capacity to disperse and find suitable habitats for metamorphosis into adult forms that then reproduce vegetatively (clonally) to form colonies in that suitable habitat. Minimal selection takes place at the clonal level, but each clone will produce many millions of larvae over its lifetime.
Extending my examples on the evolution of the camera eye that seem to have ended the Reductionism [Causality of Life] thread on why I think the so-called Intelligent Design arguments against evolution are rubbish (see Why the Argument From Design Doesn't Hold Water), I present some examples of where larval evolution has almost certainly played a major part in the origins of the vertebrate body plan (i.e., our own).
My first set of examples are provided by the fantastic diversity of larvae found in the phylum Ectoprocta (AKA Bryzoa). Ectoprocts are millimeter-sized colonial organisms that often form rapidly growing colonies in the form of calcareous crusts on all kinds of surfaces from kelp fronds to rocks. The Ectoproct figures are all from Hyman, L.H. (1959). The Invertebrates. Volume 5. Smaller Coelomate Groups Chaetognatha, Hemichordata, Pogonophora, Phoronida, Ectoprocta, Brachiopoda, Sipunculida: The Coelomate Bilateria.
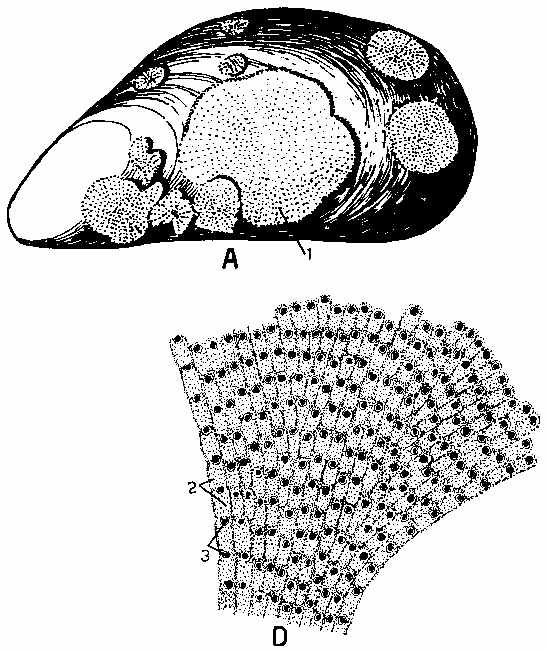
Fig. 102, p. 286: A shows the crustose colonies on a mussel shell. Note that the colonial organism grows fast enough to colonize and pretty much fill the surface during the life of the mussel shell itself. D. is a more magnified view of a colony. A single zooid lives in each box. Zooids are ciliary current feeders. Beating cilia on the tentacles draw a stream of water towards the zooid, where food particles are trapped in mucous and passed into the gut for digestion
From Fig 111, p. 305. shows a cross section through a single zooid. Callouts are as follows: 1. tentacles; 2. mouth; 3. Neural ganglion; 4. ring ceolom of the lophophore (= the collection of tentacles) base; 5. coelomic septum; 6. tentacle sheath; 7. anus; 8. vestibule; 9. diaphragm; 10. sphincter; 11. dilators of vestibule; 12 dilators of diaphragm; 13. pharynx; 14. esophagus; 15. ovary; 16. cardia; 17. stomach; 18. pyloris; 19. intestine; 20. parietal muscles; 21. cuticle; 22. epidermis; 23. peritoneum; 24. coelomocytes (closest thing to white blood cells); 25. testes; 27. funniculus; 28. retractors of lophophore. Basically, the retractors serve to instantly retract the costly lophophore into the calcareous armour of the zoecium (literally the zooid's house) and close the door. The other muscles are associated with the hydraulic system to extend the lophophore when it is again safe to feed. Adults have no mobility and grow additional zooids vegetatively.
Larvae provide the means to disperse and colonize new surfaces. The phylum has a large variety of larval forms. Some live for some time in the plankton as self-sustaining - i.e., feeding - organisms until they locate a suitable surface they can glue themselves to and metamorphose into the sedentary adult form.
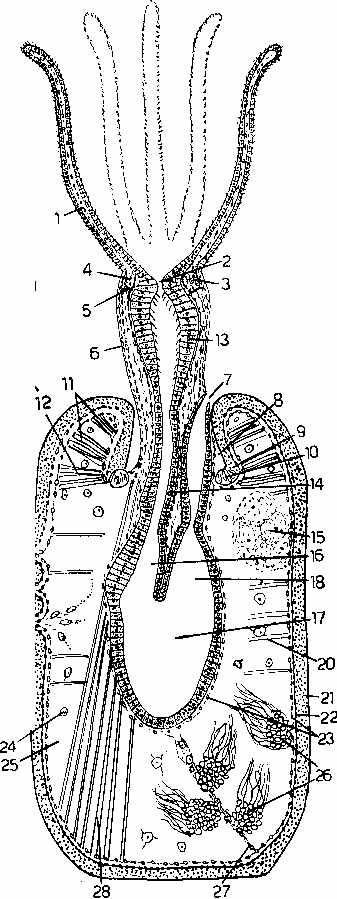
The illustration above is from Fig. 132, p. 351. showing the larva of Flustrellidra hispida, where the dorsal surface is covered by a thin shell. Callouts are: 1. apical nervous organ (sensitive to light and gravitation); 3. adhesive sac (for attachment at the time of metamorphosis); 5. shell; 12. vibratile plume (long, presumably sensory, cilia); 14. pyriform organ; 15. ciliatiary girdle (locomotion); 17. muscle strands. The similarity between this body plan and that of the most primitive molluscs is interesting.

The two illustrations above are from Fig. 133 p. 353, and respectively show young and mature larvae of Farrella. The upper illustrations are respectively the side and end views of the young larva before it forms a shell. The lower more mature larva has all the sensory and locomotory apparatus to move freely in the water column and feed itself for a prolonged time. It also has some organs to assess surfaces and to form a preliminary attachment before beginning metamorphosis. Callouts are 1. apical nervous organ; 2. sensory bristles, 3. ectoderm; 4. neuromuscular strand; 5. pyriform organ (note this is a ciliated mucous secreting sensory organ analogous - if not homologous - to a molluscan head); 6. stomodaeum (mouth); 7. stomach; 8. proctodaeum (will join with the stomodaeum to form a complete digestive tube); 9. ciliary girdle; 10. adhesive sac (perhaps analogous to the molluscan foot); 11. vestibule; 12. pharynx; 13. intestine; 14. bivalve shell; 15. vibratile plume; 16 ciliated cleft; 17. adductor muscle of valves (i.e., used to pull the two shells together like a clam); 20; coelomic cavity; 22. anus.
It should be noted that the larvae of sessile invertebrates in general have a variety of body plans that exceeds those of mature organisms in all phyla.
The next diagram illustrates the larva of a class of chordates that are probably intermediate between a lophophorate ceolomate (i.e., body plan somewhat like that of the entoprocts) and that of a very primitive fish. It is very likely that our ancestors passed through such a stage of evolution.
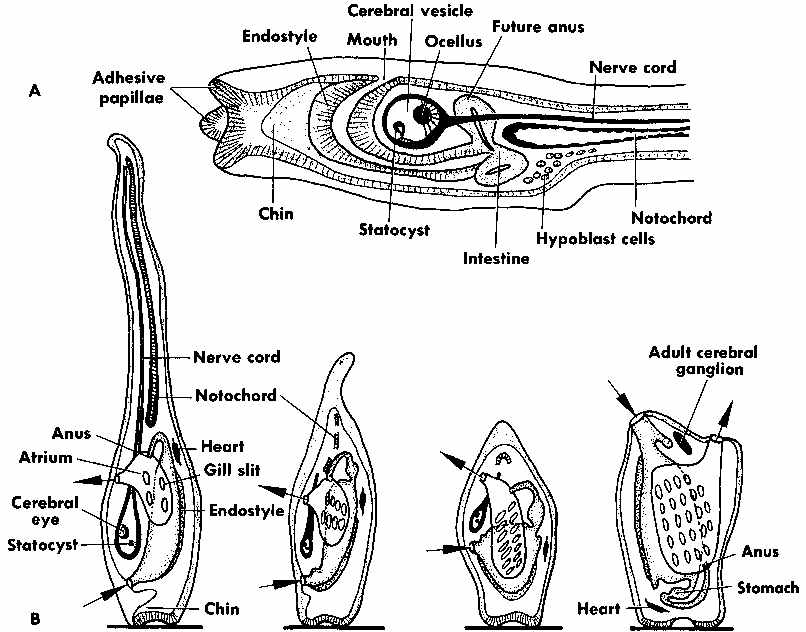
The source is Hickman, C.P. (1973). Biology of the Invertebrates. 2nd Ed., C.V. Mosby Company, St. Louis. Mo.. Fig. 18-6 from p. 716 illustrates the larva, metamorphic stages and adult form of the simple tunicate (sea squirt) Ciona.
The tunicates are still ciliary current feeders, but they have permanently moved the ciliated surfaces inside the body where they form a filter basket able to filter particles of food from the current of water the cilia pump through the body. Unlike the larvae of most other invertebrates that are microscopic and use their cilia for locomotion, the tunicate larva is larger, shaped like a tadpole, and swims like a fish using its muscular tail. By using muscular propulsion much larger larvae can swim rapidly through the water column to escape predation or find shelter. The larvae also have a dorsal nerve cord and a post-anal tail supported by a notochord (that is the embryological basis for the backbone in vertebrates) with an expanded front end including an eye and statocyst (gravity sensor). The larvae have the same basic body plan as all vertebrates do, but after metamorphosis, the colonial zooids of most of the sexually reproducing adults are little more than a vacuum cleaner filter bag with an intestine wrapped up in a cellulose sack.
However, the larval body plan of one group of tunicates has become sexually mature, and forms a major component of oceanic plankton (the larvacea). Long ago, it is likely that a similar larval neoteny occurred in our direct ancestry in the late Precambrian or Cambrian eras. Note, if the larvae are successful feeders in their own right there are obvious selective advantages to developing reproductive maturity at earlier developmental stages.
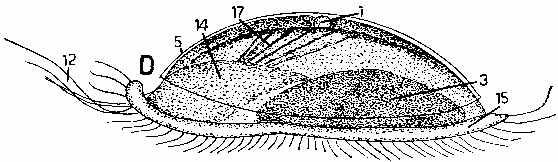
There is an interesting survivor showing what that ancestral pre-vertebrate may have looked like, with the common name of Amphioxus: The next two pictures are from Walker, W.F. (1975). Vertebrate Dissection (5th Ed), W.B. Saunders Company, Philadelphia. The first, from Figure 1-3, p. 8. shows an external view of an amphioxus. The second, from Figure 1-4, p. 8, shows a cleared and stained whole mount of a young specimen that gives a good idea of the internal anatomy.


These are fish-shaped filter feeding organisms up to 10-20 cm long or even larger at maturity that spend most of their time submerged in sand with only their mouths exposed to create the feeding current. However, if disturbed, they can erupt from the sand and swim rapidly like a fish to escape predation.
The next picture is a magnified version of the head region of Amphioxus from McFarland, W.M. et al., (1979). Vertebrate Life. Macmillan Publishing Co., Inc. New York - Fig. 2-5, p. 28. The buccal cirri, wheel organ and pharyngeal bars are all ciliated and work to keep a rapid current of water flowing through the phryngeal filter basked. The endostyle is a ciliated thyroxin secreting organ that collects food captured on the filter bars and conducts it to the gut. It is directly homologous (ancestral) to the thyroid gland of vertebrates, but here there is a close connection between the amount of food conducted to the gut and the amount of thyroid hormone secreted to control the overall metabolic rate of the organism. Amphioxus also has a small eye spot. The pharyngeal feeding system is closely similar to that of the sessile tunicates.

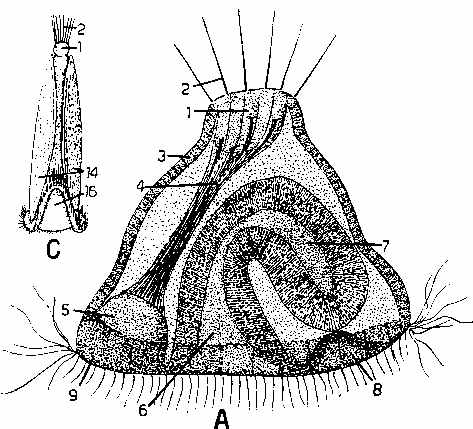
Interestingly, the relationship between Amphioxus and the vertebrates is supported, not so much by Amphioxus itself, but by one of the most primitive fish-like vertebrates, the Lamprey. The last two graphics, from Walker (loc. cit.), respectively from fig. 2-6, p. 23 and fig. 2-3, show the anatomy of a mature lamprey (a marine parasitic fish without paired fins or a true jaw) and its larva - the larvae live in fresh water streams for several years before metamorphosing into the adult. Anatomically the larval lamprey (known as ammocetes) is very similar to Amphioxus, with the exception of a substantially better brain and sense organs. The brain has three major sections as in other vertebrates, there are lateral image forming eyes and a single dorsal eye, an inner ear consisting of a sacculus (gravity sensing) and two semicircular canals (other vertebrates have three, and an hypohyseal pouch which defvelops into a primitive olfactory organ.


Obviously this is a very abbreviated abstract of a small fraction of the detail that can be brought to bear on the question of how "macro" evolution has occurred. Most of the major body plans evolved during the late Precambrian and early Cambrian when the atmosphere became rich enough in oxygen to support oxidative metabolism and the secretion of calcareous armor plating and skeletons. What most people don't realize is that there was far more time to experiment and evolve body plans in this period than there has been since the Cambrian till now.
Mayr and Gould provide the logical basis for natural selection and evolution. Even without the fossil record of mature organisms with persistent hard parts and the additional lines of evidence and explanation provided by biochemical, chromosomal and cellular genetics, the diversity of life living today provides more than enough corroboration for the factual plausibility of evolution. There is no need whatsoever for any supernatural explanations to account for the diversity of organisms that exist today.
Only those who are truly ignorant of the mechanics of heredity, population biology and the existing diversity of life could accept the kinds of specious arguments from "intelligent" design and creationist camps. My bible is the world that exists. I have poked, prodded, watched, dissected, analysed, been bittn by and otherwise interacted with this world from the limits of light microscopy to extent of community and ecosystem ecology. That is the reality, not some myths that were written down 1900 years ago by politicians and priests who probably wanted people to not think for themselves in order to better control them.
In any event, by all means read the books, and if at all possible study some invertebrate and vertebrate zoology and learn a bit about how life actually works before preaching what could or could not have happened in evolution.